|
| http://saxonylutheranlovechemistry.wikispaces.com/Chemical+Reactions |
Summary: This week in class we learned about the different types of chemical reactions. The four basic chemical reactions that can occur are: Synthesis reaction. Decomposition reaction, Single-Displacement reaction, and Double-Displacement reaction. A Synthesis reaction is a chemical reaction where two reactants make a product. A Decomposition reaction happens when a single reactant makes two products each apart of the first reactant. A Single-Displacement reaction is when one chemical in a formula switches with another element in the product. A Double-Displacement reaction is when 4 elements switch (2 Non-Metals and 2 Metals) for the product.
SP5: Using mathematics and computational thinking
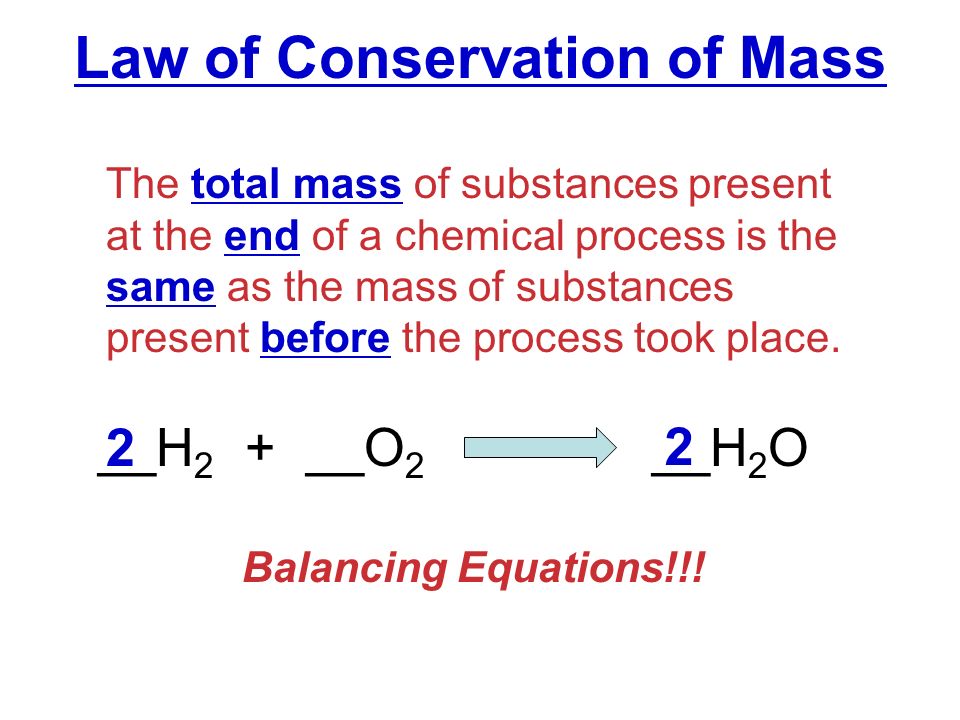
This week in class we used math calculations to balance equations then figure out what type of chemical reaction was going on. Some math-related things that we had to pay attention to were: How many of each element were in the equation, Where was each element in the equation, How did each element move from reactant to product.