|
| http://chyscience.blogspot.com/2012_08_01_archive.html |
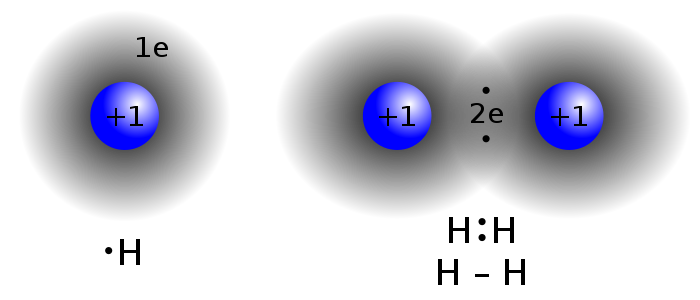
Summary: This week in class we learned about ionic bonding. Ionic bonding happens when two atoms (metal and non-metal) share an electrons. The atom with the most valence electrons which are the electrons on the outer most shell of the atom, receives an electron from the atom with lesser amount of valence electrons. Then the atoms attract because the atom that got the electron is now a negative ion and the atom that gave the electron is now a positive ion, and then the atoms bond together to make an ionic bond.
SP2: Developing and using models
This week in class we used model atoms with clay on them to represent electrons. We then were given a sheet of paper with 5 bonds we had to do. We then had to determine whether the bond was ionic or covalent. If the bond was ionic we would give the atom that had more valence electrons an electron from the other atom. If it was a covalent bond then we put the atoms on top of each other.