 |
| https://commons.wikimedia.org/wiki/File:Covalent_bond_hydrogen.svg |
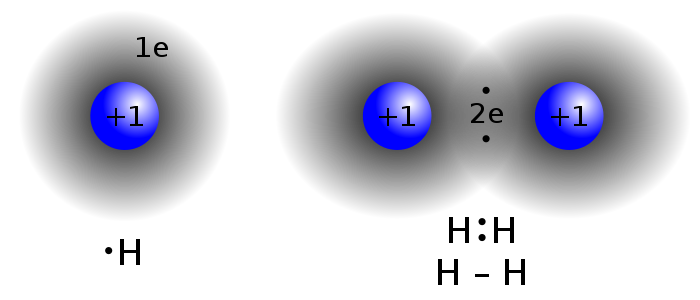
Summary: This week in class we learned about covalent bonding. A covalent bond is formed when two atoms want to take each others electrons. When an the atoms want to take an electron they get closer to the electron. When they get closer to the electron then it begins a loop where the atoms try to get closer to each others electrons bet cannot. YOu cannot have H3O because the amount of inner electrons cannot go over two and the outer electrons do no have strong enough bond to the nucleus for covalent bonds to form.
SP4: Analyzing and interpreting data
This week in class we analyzed data by getting a document with links on it and then reading what each website had to say very carefully. We also analyzed an image that showed us how electrons work in covalent bonding. We interpreted the data in a document that we were given we answered the questions on the document based off of our research
No comments:
Post a Comment