|
Backward Looking I feel like the charity that I chose for the charity fair tells a story. It resonated with me especially because when I was little I was always bullied. I feel like conveying my story through my charity is one of the best things I could do.
Inward Looking I feel like it was incredibly satisfying to tell my story through the charity I chose. It feels satisfying to finally get closure after all these years of regretting to stand up to bullies. I wanted others to not have to go through the same thing that I had had to go through.
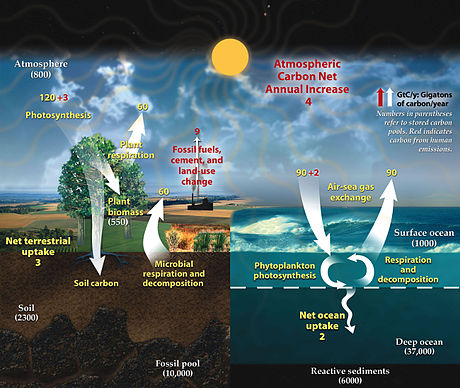
Outward Looking I did not work the way others did, because most others either did a food or handmade project. My partner and I did a digital key chain design and 3-d printed it.We still had to calculate the carbon emissions, and the cost for the materials.
Forward Looking One thing I would like to improve upon is: not relying on my teammate so much. Since my partner had the 3-d printer he designed and printed all of the products. I could only really do the work at school. Next project I will try to carry more weight in the group work field.











.svg/550px-World_Koppen_Classification_(with_authors).svg.png)